DPD As a Reference Measurement
The most common reference measurement for disinfectants is DPD. This article summarizes how DPD sensing works and what you should keep in mind when using it.
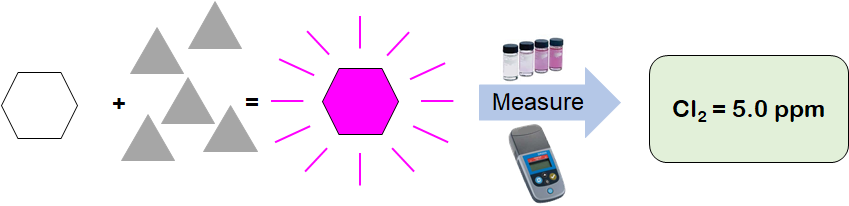
DPD (N,N Diethyl-1,4 Phenylenediamine Sulfate) is a reagent that reacts directly with disinfectants (e.g. chlorine, chloramines, chlorine dioxide, ozone, etc.) to produce a pink colored solution. The intensity of this colored solution is proportional to the concentration of disinfectant in the sample. The more disinfectant present, the darker the pink color produced. This type of measurement is commonly called a colorimetric measurement.
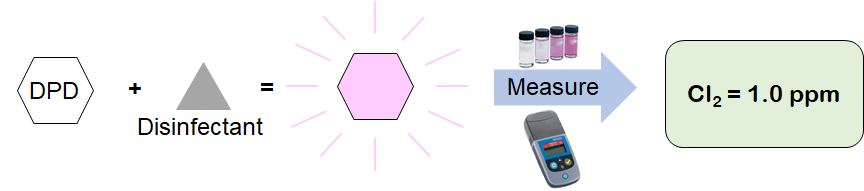
DPD measurements require a "grab sample" of process water, collected in a sample vial. The DPD reagent is then added to the vial, mixed, and inserted into a colorimeter for measurement.
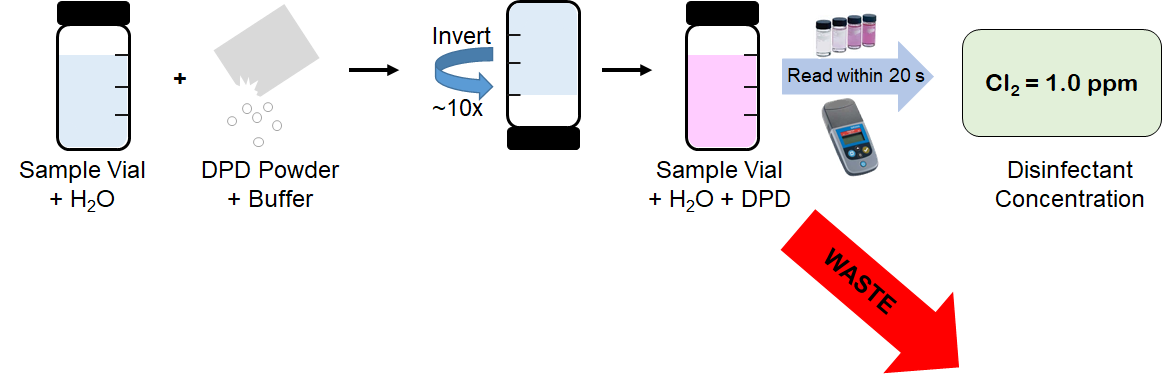
One drawback of DPD measurements is that the sample collected for measurement cannot be returned back to the process due to the addition of reagents needed. This requires the need for a chemical waste stream, adding to the lifecycle cost of colorimetric measurements.
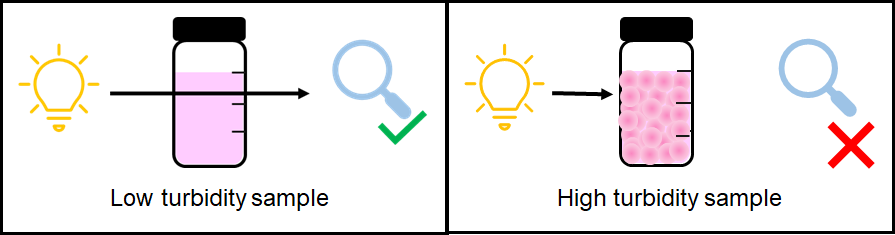
DPD measurements are also subject to several chemical and physical interferences that are commonly found in process water. If the sample contains particulate matter or is not colorless, the DPD measurement may not be accurate since it depends on light passing through the sample and being collected by the colorimeter.
The DPD reaction is also non-specific; the colored product will form when DPD reacts with free chlorine, chloramines, chlorine dioxide, ozone, and peroxides. Thus, it can be difficult to interpret DPD measurement results if there is more than one disinfectant species present. DPD will also react with metals that may be present in process waters such as manganese or iron, producing a false positive result. These chemical interferences can be difficult to control or correct for, limiting the use of DPD in many applications.
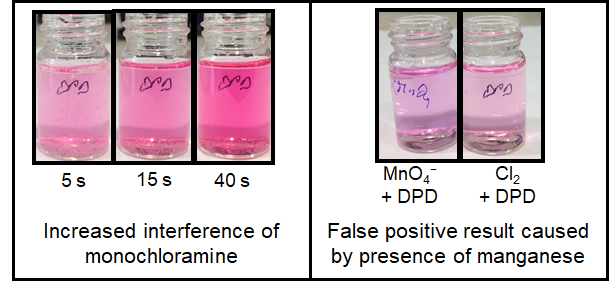
For most users, DPD measurements are used as a reference measurement that provide the "starting point" for the calibration of their amperometric sensors. A poor calibration caused by an erroneous DPD measurement will impact the quality of the amperometric measurement until the next calibration.
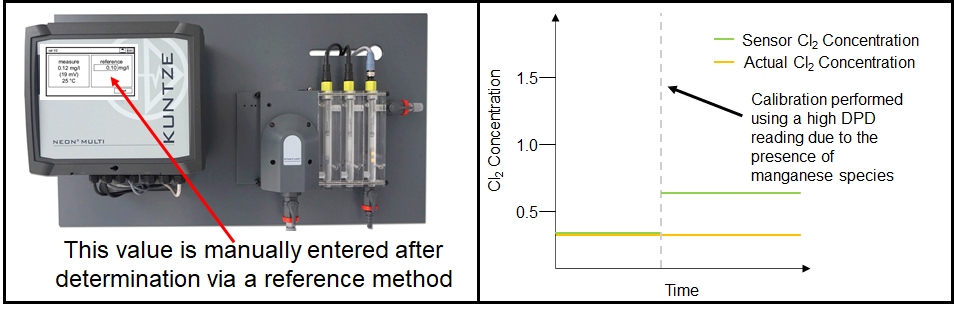
Thus, it is important to be aware of the limitations of DPD as a reference measurement, and how to correct for them if possible. Consider the conditions of your process: is your water cloudy? Does it contain any other disinfectants or interfering species? If there is a commercially-available method to eliminate an interference (e.g. a different reagent set that does not react with the interference), consider using that as your reference measurement instead. Even seemingly insignificant errors can turn into large errors in the long run.
If you aren't sure whether something in your process could be impacting your reference measurement, please contact a Kuntze representative by submitting a ticket.