Understanding Breakpoint Chlorination
This article summarizes breakpoint chlorination as it applies to systems that either contain ammonia or are using chloramination.
Breakpoint chlorination is important to understand for systems using chloramination, or in chlorination systems where ammonia might be present, such as wastewater systems.
Important Terms to Know
> Free chlorine: combination of Cl2, HOCl, and OCl-
> Combined chlorine: consists of mostly chloramines, which are formed when ammonia is added to free chlorine, yielding monochloramine (NH2Cl), dichloramine (NHCl2), trichloramine (NCl3), and organic chloramines
> Total chlorine: sum of free and combined chlorine
The graphic below depicts the chlorine residuals as a function of the added chlorine dose.
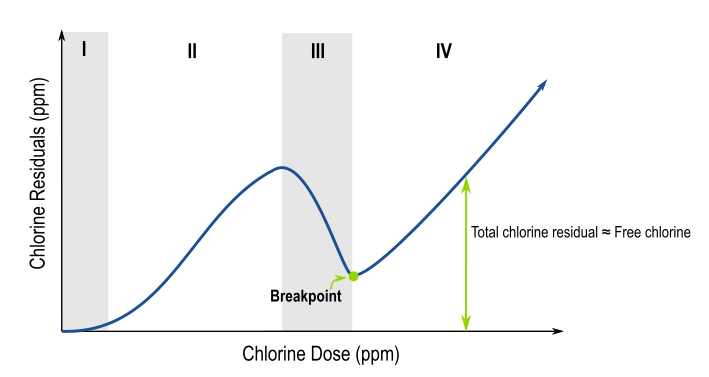
Zone I
Most of the free chlorine added in this region gets reduced by transition metals present in the
water such as iron or manganese. Total chlorine residual increases minimally with added free chlorine.
Zone II
As the dose is increased, free chlorine reacts with ammonia to form monochloramine, which has some disinfection ability. Total chlorine residual increases as more free chlorine is dosed and more chloramines form.
Zone III
In Zone III, total chlorine residual drops as more free chlorine is added to the system. Monochloramine is oxidized to dichloramine, then to gaseous trichloramine, which evaporates away. Eventually, the chlorine dose overcomes the oxidant demand in the system, called the breakpoint.
Zone IV
Once the breakpoint is reached, the chlorine residual increases roughly linearly with the added free chlorine dose. There is now a free chlorine residual present in the system for disinfection purposes.
The shape of the curve and the relative concentrations depend on characteristics of your system such as temperature, pH, and what organic species are present. If you have further questions regarding breakpoint chlorination, contact a Kuntze representative or submit a ticket.