Bare Amperometric Sensor vs. Membrane Sensor Technology
Looking to measure oxidant in an application that frequently shuts down and starts up? Want to reduce the impact of flow and pressure changes on your oxidant measurement system? Switching from a membrane-style amperometric measurement system gives you these benefits and more. Watch our new video to learn about Kuntze's bare amperometric sensors and how they outperform membrane sensor technology.
Were you looking to compare Kuntze systems to online colorimetric sensor technologies? Click here to learn more.
Bare Amperometric Sensor vs. Online Colorimetric Sensor Technology
Looking to eliminate the need for reagents for your oxidant measurement? Want a more environmentally-friendly and cost-effective measurement system that doesn't need a hazardous waste stream? Switching from an online colorimetric measurement system gives you these benefits and more. Watch our new video to learn about Kuntze's bare amperometric sensor technology and how it outperforms online colorimetric systems.
Were you looking to compare Kuntze systems to membrane sensor technologies? Click here to learn more.
Why pH Matters in a Free Chlorine Measurement
When chlorine (Cl2) is added to water, hypochlorous acid (HOCl) and the hypochlorite ion (OCl-) are formed. The term "free chlorine" refers to the combination of Cl2, HOCl, and OCl- that is present in solution. HOCl is the predominant biocidal agent, or what "kills" the pathogens that may be present.
All amperometric measurements of free chlorine, including the Kuntze measurement, measure only the presence of HOCl. HOCl is reduced on the measuring electrode, which yields a current that gets translated by the instrument to a free chlorine concentration.
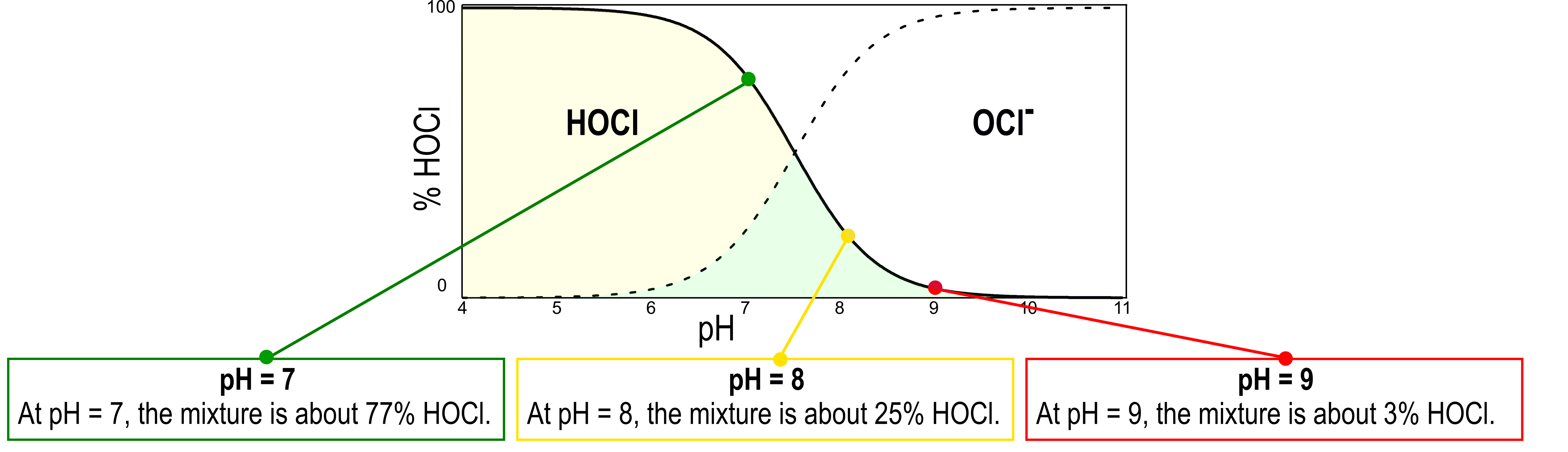
The pH of the system determines what species are present, and in what ratio, shown in the scheme on above. Above pH 8, there is a very small amount of HOCl present in solution, making amperometric detection of free chlorine a more challenging process.
At pH = 7, the mixture is about 77% HOCl, and ideal for the Kuntze measurement. At pH = 8, the mixture is about 25% HOCl. At pH = 9, the mixture is about 3% HOCl. Measurement at this pH will be challenging. Contact a Kuntze representative to discuss possible changes to your measurement system.
