Kuntze Instruments USA Presents at AWT Annual Convention and Exposition

The Kuntze Instruments USA team exhibited a booth at the Association of Water Technologies (AWT) Annual Convention and Exposition in Providence, RI September 22-25. Per the AWT website, the convention is “…where water treatment professionals and industry partners gather to exchange time, resources, strategies, solutions, and more.” During the exposition, the team had the opportunity to showcase Kuntze’s complete disinfection measurement solutions to both end users and distribution companies in the water treatment industry. Valuable knowledge and experience was shared, new connections were made, and potential opportunities for Kuntze measurement solutions were realized.
The convention also featured several educational sessions covering topics such as boiler water, polymers, business and operations, Legionella, cooling water, and general water treatment. As a part of the cooling water track, Customer Success Manager Lacey Beck presented her paper “Novel Bare Amperometric Sensors to Provide Accurate Stabilized Bromine Dosing in Cooling Towers.” Written as a collaboration with Pat Carew at Enerstar Inc., the paper outlines the first demonstration of a bare amperometric sensor to measure and directly control stabilized bromine products industrial cooling towers. Pat installed a Kuntze Krypton® DIS system with a Zirkon® DIS Total sensor on a 200-ton cooling tower treated with bromine and sulfamic acid. You can read the results of the study here: View Study.
Kuntze’s bare amperometric sensors are uniquely suited for cooling tower applications due to their rugged and robust design and their highly interpretable measurement signal. To learn more about how a Kuntze measurement system could fit into your cooling tower, check out this case study or contact a Kuntze representative.
Innovative Kuntze Analyzers Save $16,350 Operation Expenses per Year in an Alberta Surface Water Drinking System
Background
A public surface drinking water system (DWS) located in Alberta, Canada operates two level 2 drinking water treatment plants, one level 3 drinking water treatment plant, and three drinking water storage systems. The DWS used both a membrane-covered amperometric sensor and an on-line colorimetric sensor to control and monitor free chlorine residual levels in their drinking water systems.
Challenge
Both analyzer systems required significant preventative maintenance, the need for replacements parts, and the purchase of consumables. The DWS was also concerned that the measurements of the membrane-covered amperometric sensor would be influenced by bubbles, which occurred frequently in their system.
Kuntze Solution
ClearTech suggested the installations of Kuntze Krypton® Multi analyzers to measure and control the disinfectant residuals. The DWS agreed to trial the Kuntze analyzer and installed it at the distribution system header right next to the existing membrane sensor. The Krypton® Multi system also featured Kuntze’s patented ASR® automatic sensor cleaning technology, which was set-up for automatic cleaning once per week to prevent the sensor from fouling. The membrane sensor of the competitive system was manually cleaned once per month.
The trial was conducted for one month in September 2020. The operators used a handheld colorimeter taking manual DPD reference measurements five days per week. The free chlorine residual target range was 1.0 to 1.5 ppm, and the reference measurements were compared with the readings of both the Krypton® Multi system and the analyzer with the membrane sensor.
Results
While both analyzers were able to stay in the residual target range, the Krypton® Multi system had on average less deviation from the reference measurements providing more accurate readings.
Conclusions
The Kuntze Krypton® Multi system became the preferred technology to monitor and control the free chlorine residuals while significantly reducing the preventative maintenance requirements. The Krypton® Multi system requires no consumables or reagents generating significant cost savings for the drinking water system.
Recently the DWS replaced two on-line colorimetric analyzers with two Kuntze Krypton® DIS analyzers at one of their 750 cubic meter storage tanks to measure the free chlorine residuals at the inlet and discharge points. The DWS reported following reasons for their decision:
- Reduced installation and start-up time - commissioning is much easier and faster due to the plug-and-play nature of the Kuntze analyzer
- Reduced waste - sampling water can be returned into the well water storage tank, eliminating the need to discharge the sampling water of the on-line colorimetric analyzer to a sanitary sewer (4 m3/day or 1,500,000 liters/year). This water system purchases the water from another municipality at a rate of $3.15m3; savings of $4,725 per analyzer per year.
- Increased safety - no reagents are needed to create the measurement; savings of $1,920 per year per analyzer
- Reduced maintenance time - elimination of internal preventative maintenance service hours and external analyzer recertification – total savings/year = $1,530 per analyzer
- Monthly manual sensor cleaning (10-12 operator hour savings/year - $330/unit)
- Annual recertification of analyzer by manufacturer ($1,200/unit)
Replacing the two on-line colorimetric analyzers with the two Kuntze Krypton® DIS analyzers has generated total savings of $16,350 per year.
Bare Amperometric Sensor vs. Membrane Sensor Technology
Looking to measure oxidant in an application that frequently shuts down and starts up? Want to reduce the impact of flow and pressure changes on your oxidant measurement system? Switching from a membrane-style amperometric measurement system gives you these benefits and more. Watch our new video to learn about Kuntze's bare amperometric sensors and how they outperform membrane sensor technology.
Were you looking to compare Kuntze systems to online colorimetric sensor technologies? Click here to learn more.
The Purpose of Calibration
Amperometric sensors work by taking an electrical signal and translating it to a concentration. The graphic below illustrates this process.

First, the analyte of interest (chlorine, chlorine dioxide, ozone, etc.) gets reduced on the measuring electrode of the sensor. This generates a raw electrical signal, measured in millivolts (mV). This signal gets processed by the Neon® analyzer, which uses your pH, calibration slope, temperature, and other parameters to turn the mV signal into a concentration value, usually measured in ppm or ppb. This concentration is what gets displayed on the Neon® unit.
In order for the analyzer to make the conversion between an electrical signal to a concentration, a calibration must be performed. A calibration tells the analyzer "an electrical signal of ___ mV corresponds to a disinfectant concentration of ___ ppm" where the disinfectant concentration is measured with a reference method such as DPD. This process is shown below.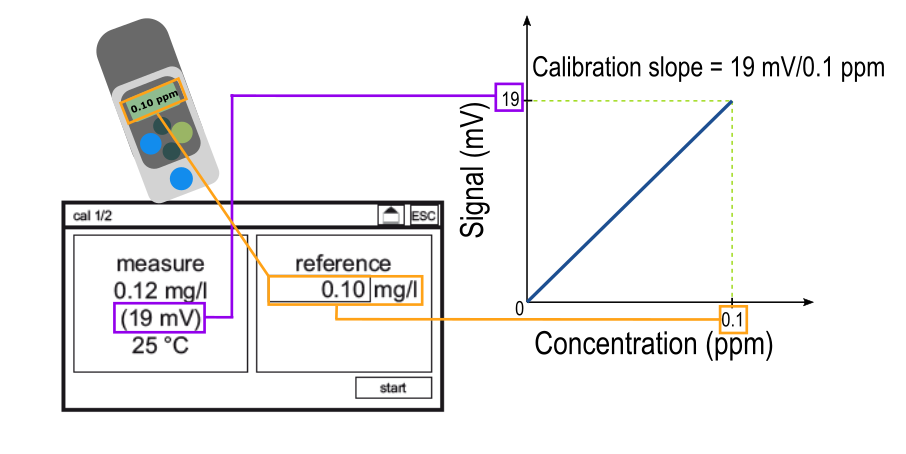
Inputting a reference value on the calibration screen generates a calibration slope. For each measuring range, there is an "ideal" slope, which you can learn more about here. The calibration slope is the tool that the analyzer uses to translate between an electrical signal and a concentration. Thus, the quality of the calibration determines the quality of the measurements made after the calibration is performed. The graphic below depicts the difference between a "good" and a "bad" calibration.
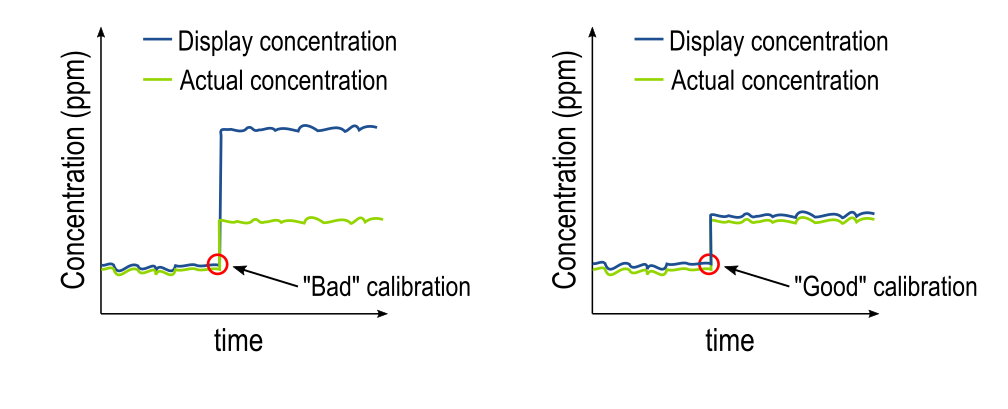
The plot on the left shows the effect of a "bad" calibration, where the reference value input was much higher than the actual disinfectant concentration, which caused a false high reading on the display. The false high reading from the reference measurement could have been caused by many factors, such as sampling errors, chemical interferences, or physical interferences, which are all common when using DPD as a reference. You can learn more about the limitations of DPD as a reference measurement here.
The plot on the right shows the effect of a "good" calibration, where the reference measurement matched well with the actual disinfectant concentration. To achieve a good calibration like the plot on the right, try taking three reference measurements back-to-back and average them together. Always be sure to follow the instructions of your reference measurement system to get the best results.
Calibration is very important to get accurate concentration readings. If you have any questions about calibration or how it works, contact a Kuntze representative or submit a ticket.
Bare Amperometric Sensor vs. Online Colorimetric Sensor Technology
Looking to eliminate the need for reagents for your oxidant measurement? Want a more environmentally-friendly and cost-effective measurement system that doesn't need a hazardous waste stream? Switching from an online colorimetric measurement system gives you these benefits and more. Watch our new video to learn about Kuntze's bare amperometric sensor technology and how it outperforms online colorimetric systems.
Were you looking to compare Kuntze systems to membrane sensor technologies? Click here to learn more.



