Why pH Matters in a Free Chlorine Measurement
When chlorine (Cl2) is added to water, hypochlorous acid (HOCl) and the hypochlorite ion (OCl-) are formed. The term "free chlorine" refers to the combination of Cl2, HOCl, and OCl- that is present in solution. HOCl is the predominant biocidal agent, or what "kills" the pathogens that may be present.
All amperometric measurements of free chlorine, including the Kuntze measurement, measure only the presence of HOCl. HOCl is reduced on the measuring electrode, which yields a current that gets translated by the instrument to a free chlorine concentration.
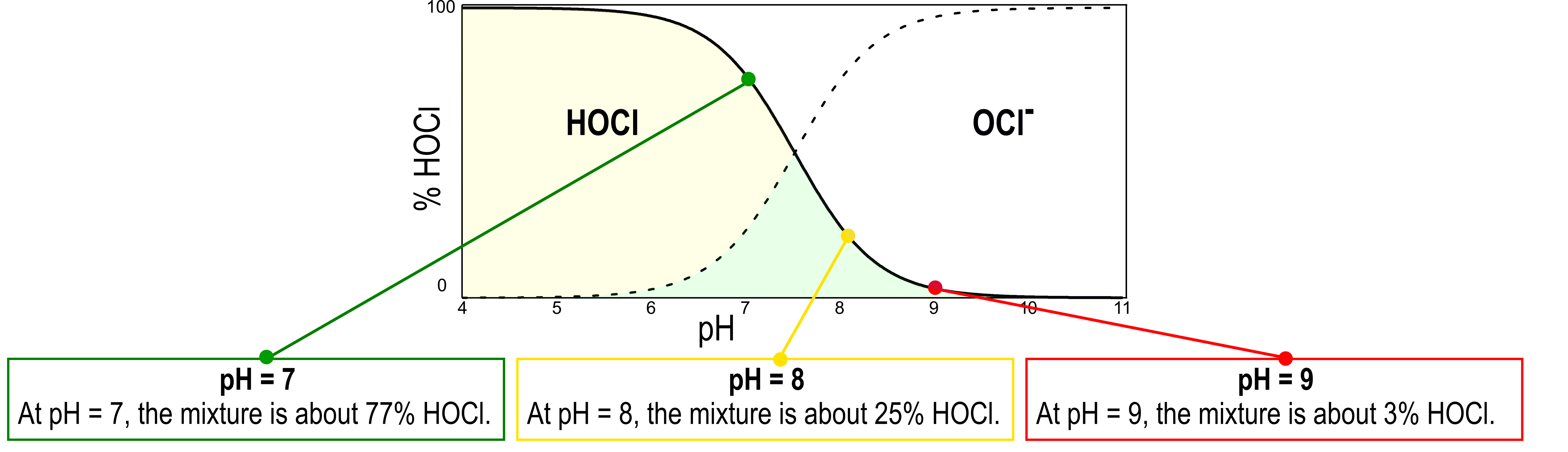
The pH of the system determines what species are present, and in what ratio, shown in the scheme on above. Above pH 8, there is a very small amount of HOCl present in solution, making amperometric detection of free chlorine a more challenging process.
At pH = 7, the mixture is about 77% HOCl, and ideal for the Kuntze measurement. At pH = 8, the mixture is about 25% HOCl. At pH = 9, the mixture is about 3% HOCl. Measurement at this pH will be challenging. Contact a Kuntze representative to discuss possible changes to your measurement system.
Understanding Compliance in Chlorine Measurements
Understanding compliance tolerances is important in assessing the health of your chlorine measurement system. Most systems follow the guidelines set forth in USEPA 334.01, which states that, when compared to a reference sample, "Analyzer reading [must be] within ± 0.1 mg/L or ± 15% (whichever is larger) of grab sample measurement."
Historically, chlorine measurements were made using colorimetric techniques, until amperometric technology became more reliable and popular. Since 2009, the EPA has judged the accuracy of amperometric technology using colorimetric techniques because they are so well defined. USEPA 334.0 builds in tolerances to account for inherent fluctuations and measurement errors that are present in both colorimetric and amperometric technologies.
Determining which set of tolerances you will use depends on your chlorine concentration. To demonstrate this, we simulated some data, shown below. The analyzer signal is shown in blue, while reference measurements are shown by green circles. The error bars on the reference measurements show either ± 15% (left plots) or ± 0.1 ppm (right plots) of the reference value. To select the correct tolerance criterion, we will look at the relative size of the error bars on the circles.
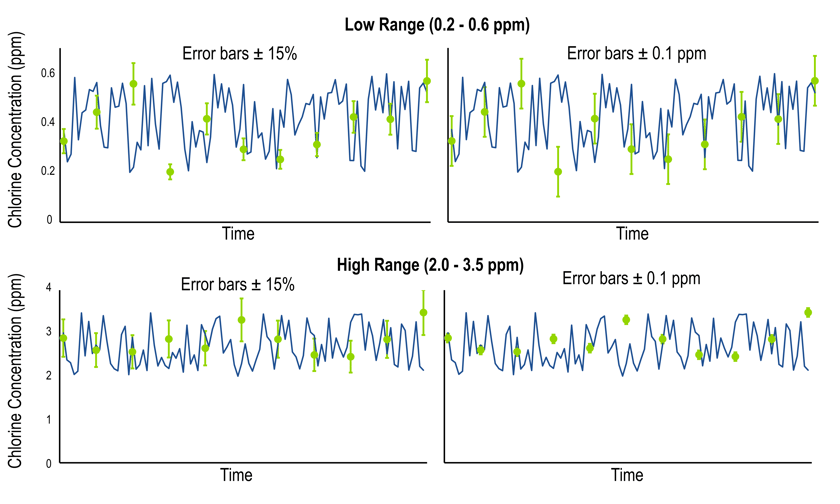
In the low concentration range (shown above), the ± 0.1 ppm criterion is the accepted tolerance because its error bars are larger. In the high concentration range (shown below), the ± 15% criterion is the accepted tolerance because its error bars are larger. If you have questions about compliance and how it applies to your system, please contact a Kuntze representative.
Understanding Breakpoint Chlorination
Breakpoint chlorination is important to understand for systems using chloramination, or in chlorination systems where ammonia might be present, such as wastewater systems.
Important Terms to Know
> Free chlorine: combination of Cl2, HOCl, and OCl-
> Combined chlorine: consists of mostly chloramines, which are formed when ammonia is added to free chlorine, yielding monochloramine (NH2Cl), dichloramine (NHCl2), trichloramine (NCl3), and organic chloramines
> Total chlorine: sum of free and combined chlorine
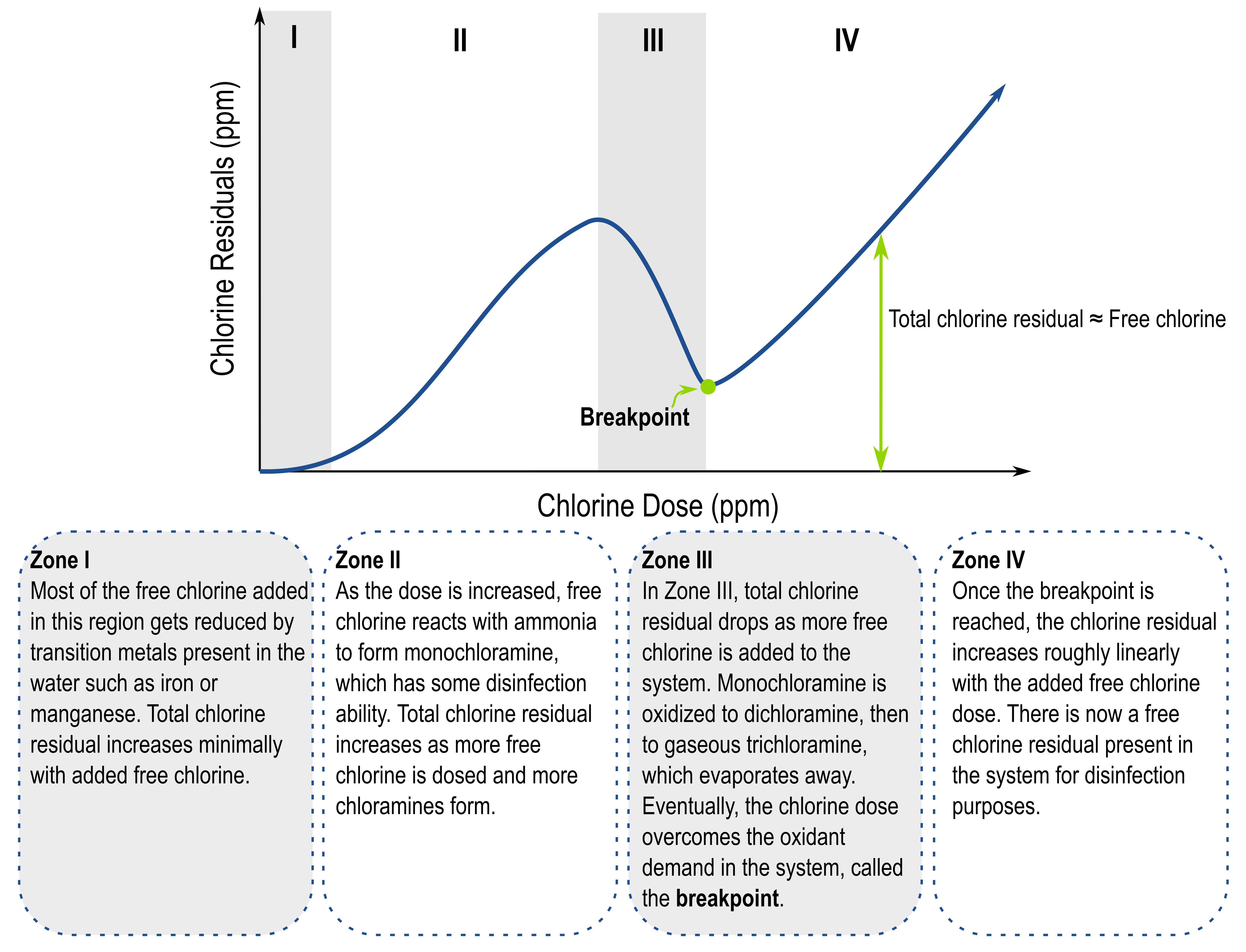
The shape of the curve and the relative concentrations depend on characteristics of your system such as temperature, pH, and what organic species are present. Still have questions? Contact a Kuntze representative to learn more.
Measuring Chlorine in Dechlorination Applications
Dechlorination is the removal of chlorine from water within a process before discharge. Most dechlorination systems use chemical reducing agents such as sulfur dioxide, bisulfite, or metabisulfite, which react with chlorine and convert it to chloride ions. Different regulations require a certain maximum chlorine residual of less than 0.5 ppm present before the water can be discharged to the environment.
Applications that regularly see 0 ppm disinfectant conditions have proven to be especially difficult for many measurement systems. Without disinfectant present, most applications see an increased presence of biogrowth, which covers electrodes, coats membranes, and clogs flow cells. Chemical reducing agents commonly used for dechlorination can interfere with the chlorine measurement.
Kuntze's measurement systems are designed to minimize influences caused by biogrowth. For the Zirkon® DIS probe (free chlorine) our patented ASR® automatic sensor cleaning technology can be triggered up to once a day to defend against biogrowth buildup. Both the Zirkon® DIS probe and the Zirkon® DIS Total probe (total chlorine) are robust enough to withstand manual cleaning as needed.
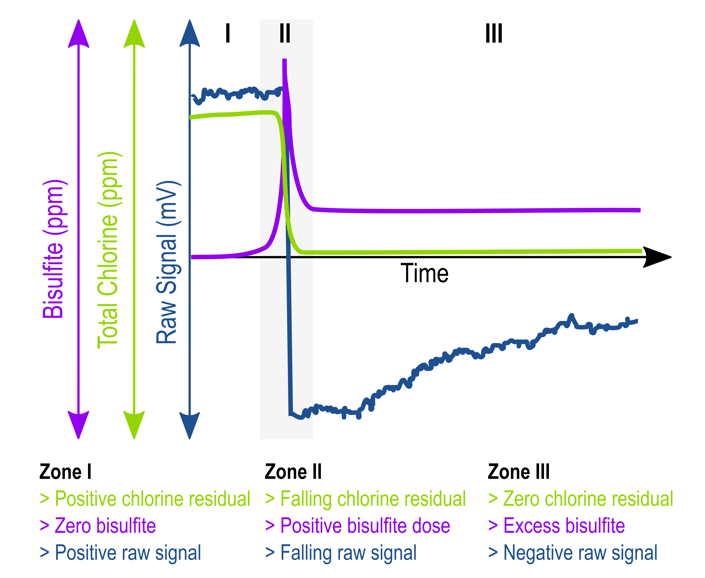 The presence of reducing agents will cause the Zirkon® DIS Total probe to show a negative mV raw signal. This is normal and shows that you have a 0 ppm total chlorine residual (see the plot above). When operating at such low chlorine concentrations, it is important to understand the limitations of your reference measurement. Most reference systems are not capable of measuring less than 0.02 ppm.
The presence of reducing agents will cause the Zirkon® DIS Total probe to show a negative mV raw signal. This is normal and shows that you have a 0 ppm total chlorine residual (see the plot above). When operating at such low chlorine concentrations, it is important to understand the limitations of your reference measurement. Most reference systems are not capable of measuring less than 0.02 ppm.
Free vs. Total Chlorine
Free chlorine is the combination of Cl2, HOCl, and OCl-. Combined chlorine is formed when ammonia is added to free chlorine, yielding chloramines (mono-, di-, tri-, organic). Total chlorine is the combination of free and combined chlorine in solution.

For applications that contain no ammonia, a free chlorine measurement is appropriate. For applications where ammonia or chloramines might be present (e.g. wastewater and drinking water), a total chlorine measurement is appropriate.
The main difference is which probe you will use. The Kuntze Zirkon® DIS is for a free chlorine measurement, and measures the presence of only HOCl. The Kuntze Zirkon® DIS Total (right sensor shown) measures the presence of both HOCl and OCl-, which allows the analyzer to give a total chlorine measurement.
Be sure your reference measurement is configured to measure the correct parameter, and that you have purchased the correct reagents to do so. The free chlorine measurement works best below pH 8.5, while the total chlorine measurement is capable of going up to pH 10. If you have any further questions, please contact a Kuntze representative.
